
31.03.23
Hygiene monitoring: the agony of making the right choice
Hygiene monitoring refers to the systematic monitoring of measures aimed at preserving health. This practice is applied in various industries such as pharmaceuticals, biotechnology, cosmetics, and food production. Additionally, it is employed in settings like hospitals, pharmacies, commercial kitchens, and other areas with high hygienic requirements to oversee the hygiene of employees, products, and processes.
Summary
Hygiene monitoring is a simplified term for the general monitoring of measures aimed at maintaining health. This applies in particular to various microbiological contaminants, which can lead to direct and indirect infectious diseases, chronic illnesses of all kinds, allergic reactions and epidemics.
Microbiological hygiene checks of various clean rooms, such as in pharmaceutical production, operating theaters and quarantine rooms, food production, public areas and other hygienically sensitive areas, serve to verify the quality of your own hygiene management with tangible data through regular monitoring and, if necessary, to correct it.
Ultimately, hygiene monitoring confirms the quality assurance of products and services of all kinds, as well as efforts to ensure the safety of employees, patients, visitors and consumers, not to mention the immediate surroundings and the general environment.
Hygiene monitoring
Based on the EU standard EN 17141, “Cleanrooms and associated cleanroom areas – Biocontamination control”, – formerly EN ISO 14698-1:2003 and EN ISO 14698-2:2003 – a mix of sedimentation plates, swab sampling, active airborne microbial measurements and surface examinations using swab plates is used for the microbiological hygiene monitoring of production facilities in regulated areas.
The regulations require regular monitoring to be carried out at a frequency that is high enough to detect any deviations at an early stage and to ensure that the data collected is statistically significant. The frequency of monitoring depends on factors such as the GMP class of the cleanroom, the risk assessment of the production process, the cleanroom design, interactions with people in the immediate vicinity and existing historical data, which should be evaluated by trained experts.
Every cleanroom operator is responsible for determining, justifying and regularly adjusting the frequency of monitoring. Hygiene monitoring is used in the pharmaceutical, biotech, cosmetics and food industries as well as in hospitals, pharmacies, commercial kitchens and other hygienically demanding areas to monitor employee, product and process hygiene.
The right measuring method: spoiled for choice
Various measurement methods are available for obtaining the required measurement data. Depending on the type of germs or infectious particles to be measured, the sampling volume, frequency, observation period and suitable method should be selected and carried out by specialists and the data carefully evaluated and communicated. In addition to classic culture-based microbiological measurement methods or direct and indirect detection methods of direct and indirect hygiene parameters, rapid microbiological methods and alternative microbiological real-time detection methods are also available.
In a specialized laboratory or “on site” at the customer’s premises
In addition to highly specialized laboratories for microbiological sample or parameter analysis and the very specific evaluation of the samples obtained during the inspections, further technologies are available with “on-site” microbiological analysis methods for a significantly faster and more targeted correction of existing hygiene measures.
While highly specialized central laboratories can provide very good qualitative and quantitative results with high accuracy, low detection limits and excellent specificity down to the DNA/genome and protein sequence level, the simpler methods of on-site measurements are usually less accurate and specific. However, the results are available much more quickly and corrective measures can be taken much faster. This is crucial in all areas where it is not possible to wait several hours or days for a result.
A combination and expertise of specific laboratory evaluations and faster – often less precise – on-site measurement methods ensures a high quality of the entire service chain – from effective, fast and correct data collection and evaluation, a specialist analysis of the samples, to expert conclusions and the suggestion of hygiene control measures to be taken.
In general, a self-optimizing system is proposed for microbiological control on a continuous basis, which identifies, implements, controls or monitors and adapts. This is a classic cyclical process of continuous improvement in which the principles: Plan – Do – Check – Act (PDCA), apply.
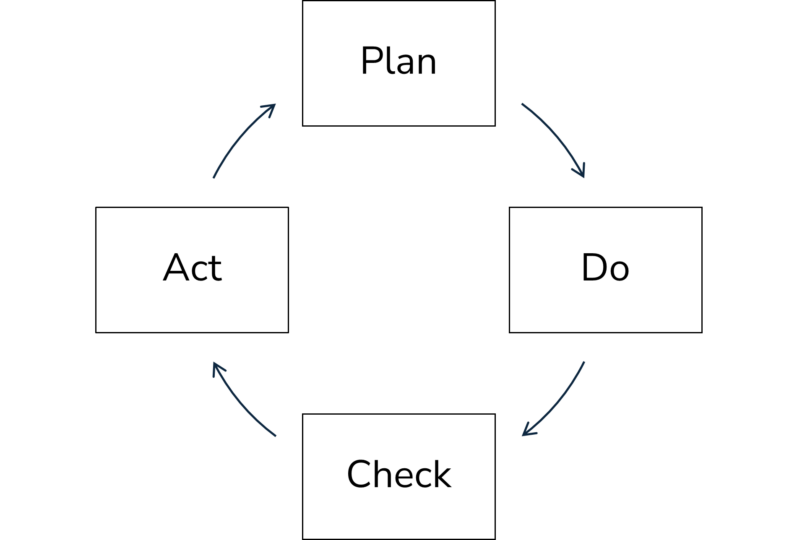
When creating and planning a suitable hygiene solution for an operational microbiological control system, adapted bioanalysis is the central cornerstone. All potential sources and pathways of microbiological contamination must be identified and appropriate solutions planned and tested on site or in the laboratory. During implementation and application as well as the other phases of continuous improvement, rapid on-site measurement methods in particular can help to monitor, check and maintain a selected concept of measures or to correct and thus improve it accordingly.
In summary, the advantage or benefit of an adapted biocontamination control consists of a combination of different factors:
- Selection of appropriate on-site analyses and/or central laboratory confirmations through adapted and optimized microbiological measurement methods; this as a combination of “fast on-site” and “accurate and specific” in the laboratory.
- Data analysis and risk assessment as well as creation of a specific and system-optimized monitoring plan
- Expert recommendation in the event of deviations and taking appropriate corrective measures
- Above all, however, short reaction times are crucial for carrying out the necessary analyses as well as corrective, decontamination and hygiene work before major damage is caused to people, products or the environment.
Specific implementation in the various industries and the healthcare sector
A wide range of different guidance is available, particularly for the pharmaceutical, biopharmaceutical, medical device and other life science industries, as well as healthcare and hospitals and related applications. What all sectors and industries have in common is that they have and/or use highly relevant and controlled areas in terms of hygienic purity or cleanliness.
In the regulated pharmaceutical and biopharmaceutical production industry, there are already numerous applicable standards and regulatory guidelines. These include the EU Annex 1 GMP guidelines for the manufacture of sterile drugs and the FDA guidelines for aseptic manufacturing. European and US pharmaceutical regulations also contain some guidance on certain related topics. Numerous other documents and technical articles are available from industry associations including the Parenteral Drugs Association (PDA), the International Society of Pharmaceutical Engineering (ISPE) and the Pharmaceutical Healthcare Sciences Society (PHSS).
In the healthcare and hospital sector, EU guidelines including tissue and blood guidelines are available for specialized areas and similar cleanliness controlled areas. National standards and guidelines are available for specialized operating rooms, isolation wards and wards for immunocompromised patients as part of infection control. In addition, aseptic drug compounding areas in hospital pharmacies, radiopharmacy pharmacies and specialized laboratories such as stem cell laboratories typically use guidance documents from the life sciences industry.
While regulations and standards for the risk management of medical devices such as EN ISO 14971 are available, there is usually less guidance available for the microbiological control of other areas controlled for general cleanliness.
Even though regulations and standards are available in the food industry and related consumer goods sectors, for example for food, beverages and cosmetics, there is insufficient guidance available on microbiological control in areas controlled for cleanliness.
Literature
- DIN EN 17141:2020: Reinräume und zugehörige Reinraumbereiche – Biokontaminationskontrolle
- DIN EN ISO 14698-1:2003: Reinräume und zugehörige Reinraumbereiche – Biokontaminationskontrolle – Teil 1: Allgemeine Grundlagen
- DIN EN ISO 14698-2:2003: Reinräume und zugehörige Reinraumbereiche – Biokontaminationskontrolle – Teil 2: Auswertung und Interpretation von Biokontaminationsdaten
- EN ISO 14644-1:2015: Reinräume und zugehörige Reinraumbereiche – Teil 1: Klassifizierung der Luftreinheit anhand der Partikelkonzentration
- EN ISO 14644-2:2015: Reinräume und zugehörige Reinraumbereiche – Teil 2: Überwachung zum Nachweis der Reinraumleistung bezüglich Luftreinheit anhand der Partikelkonzentration
- EN ISO 14644-3:2005: Reinräume und zugehörige Reinraumbereiche – Teil 3: Prüfverfahren
- EN ISO 14644-4:2001: Reinräume und zugehörige Reinraumbereiche – Teil 4: Planung, Ausführung und Erst-Inbetriebnahme
- EN ISO 14644-5:2004: Reinräume und zugehörige Reinraumbereiche – Teil 5: Betrieb
- EN ISO 14644-7:2004: Reinräume und zugehörige Reinraumbereiche – Teil 7: SD-Module (Reinlufthauben, Handschuhboxen, Isolatoren und Minienvironments)
- Enzler h-tec Einstleistungen – Angewandte Hygienetechnologien
- ISO 14971, Medical devices – Application of risk management to medical devices
- Point H.A.C.C. (HACCP) system and guidelines for its application. 1995 Codex Alimentarius Commission. Alinorm 97/13. Annex to Appendix II. Joint FAO/WHO Food Standards Programme. Food and Agricultural Organization of the United Nations, Rom, 1995.
- European Commission EudraLex „The Rules Governing Medicinal Products in the European Union“, Volume 4.
- EU Guidelines for Good Manufacturing Practice, Medicinal Products for Human and Veterinary Use, Annex 1 – Manufacture of Sterile Medicinal Products, 25. Nov. 2008
- FDA Guidance for Industry – Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice. September 2004.
- IS0 18593:2018: Mikrobiologie der Lebensmittelkette – Horizontales Verfahren für Probenahmetechniken von Oberflächen



Tell us what you think
Be the first to comment this post.
You must be logged in to leave a comment.