12.07.23
Rouging: deposits in pure water systems
Coatings and deposits on stainless surfaces, such as those found in the pharmaceutical, food and biotechnology industries, can be attributed to a variety of causes. Microbial contamination, rouging, blacking and corrosion are some of the main causes of these unwanted deposits.
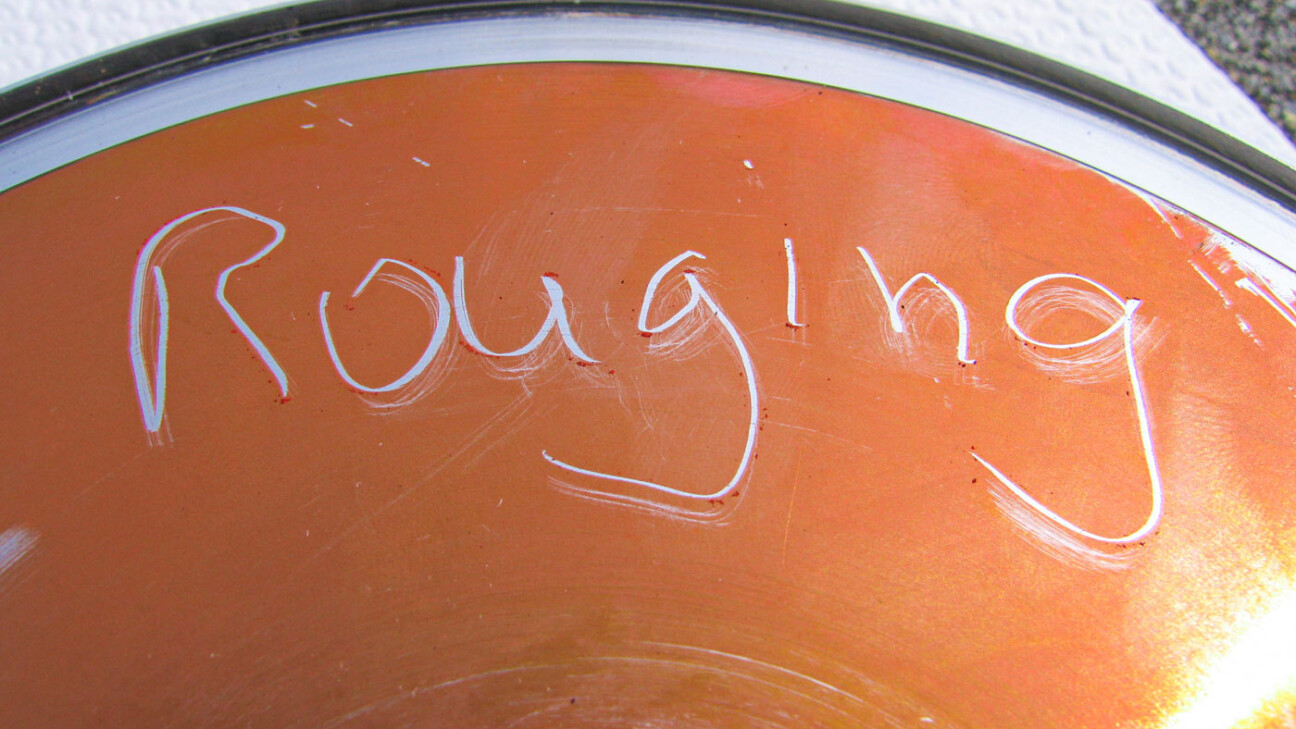
Even on carefully maintained stainless surfaces, undesirable coatings and deposits can form, especially in pharmaceutical, biotechnology and food production facilities. These deposits can impair surface quality, lead to particle contamination, promote the growth of biofilms and make cleaning more difficult. Various causes are identified in this context:
- Contamination of water piping systems due to microbial contamination – the best known contamination is the formation of biofilms
- Rouging
- Blacking
- Corrosion tensions
Biofilm & Microbial Contamination
Bacteria in moist environments adhere to the surface by secreting a slimy, glue-like substance known as extracellular polymeric substances (EPS). This may be a single bacterial species or multiple bacterial species, but may also include fungi, algae, yeasts, protozoa, or other microorganisms. The main causes of biofilm generation are as follows:
- Material (e.g. filters, hoses, pipes sealing material etc.)
- Personnel (largest source of contamination through clothing, aseptic behavior)
- room environment due to cleaning procedures
- Measuring equipment


Rouging
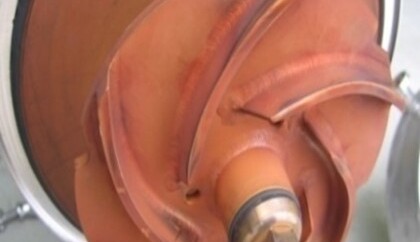
The term “rouging” refers to changes or damage to the passive layer. The phenomenon of rouging occurs preferentially in hot ultrapure water and ultrapure steam systems. It can be assumed that the very ion-poor water has a strong “suction effect” on components of the material alloys, mostly stainless steels, and thus wants to reduce their ion-poverty. With increasing temperature (> 60°C), the shift in the carbonic acid equilibrium leads to a lowering of the pH value and the oxygen depletion to a reduction in the electrochemical potential. These phenomena cause a change in the thermo-dynamic equilibrium and consequently weaken the passive film. The oxidation process produces a thin coating of iron oxide Fe2O3, also known as hematite, on the surface of the stainless steel.
In practice, affected systems show discoloration that can range from reddish-brown to purple to light blue. These discolorations usually manifest themselves as thin coatings that are either adherent or easily wiped off and sometimes even exhibit chalky properties. Depending on the material or temperature, rouging discoloration will manifest differently.
The three categories of rouging discolorations are:
| Rouging Category | Description |
| 1 | Red discoloration (deposited “flash rust”), in ozone systems violet and blue discoloration |
| 2 | Superficial formed yellow to orange discoloration |
| 3 | Dark brown deposit formation |
Blacking
Blacking refers to the significantly thicker deposits that can form in pure steam systems at temperatures between 100 and 140°C with increasing operating time. These matt black coatings consist mainly of firmly adhering magnetite layers (Fe3O4). Depending on the exposure time and conditions, different surface states develop. Initially they are slightly transparent and can then grow into chalky surface structures. The high temperatures of the steam favor the formation of these coatings. Oxidation of the iron also plays a decisive role in the case of blacking. Defective chromium oxide layers promote the formation of deposits.

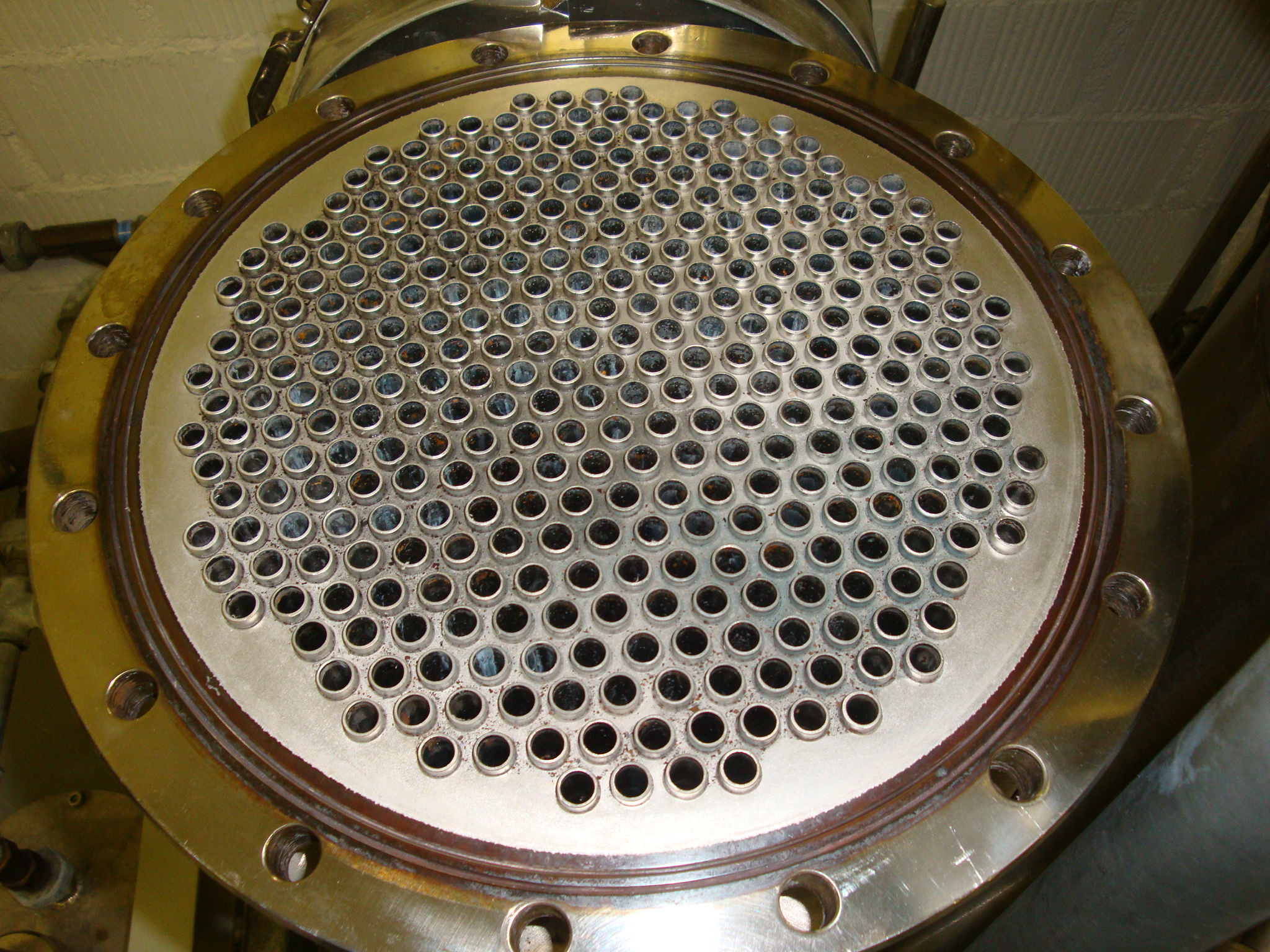
Corrosion and rust formation
In fact, even stainless steels (STNR) can suffer serious corrosion damage if used improperly. In addition to the right choice of material and proper processing, the use of corrosion protection measures is also of crucial importance. A large proportion of the corrosion damage that occurs worldwide every year could be avoided if a suitable design solution were chosen and knowledge of corrosion and corrosion protection were properly applied. Corrosion products can severely contaminate components and systems, lead to functional failures and also cause severe product contamination. Therefore, the removal of corrosion deposits and products is a serious task.
Summary
Each of these causes has specific characteristics and effects on the surfaces. In order to ensure efficiency and hygiene in production plants, it is crucial to understand the causes and take appropriate measures to prevent and clean these deposits. Would you like to learn more about passivation of stainless steel?
The following article explains the chemical oxidation process of passivation: Passivation of stainless steel | Hygieneforum.ch